Recycling
Contents
[hide]Recycling of early productive nanosystem products
Technology level I
Most materials used in early bio based productive nanosystems (naturally occurring and or artificially synthesized organic molecules -- e.g. DNA) are completely bio degradable. Recycling can be done by nature (composting).
Possible problems may be:
- waste molecules from medical applications causes hormone like effects (a known and already existing problem)
- accumulation of artificially synthesized persistent organic molecules in the environment (TODO: reference related video) (thy are called persistent organic pollutants or POPs). Note that the volumes of waste molecules produced in the early stages of APM probably won't be very high. So low toxicity POPs may not be much of an issue at first if by the time when the production volumes explode other less problematic materials with superior mechanical properties (bio-minerals) already have taken take over.
Technology Level II
Bio-minerals that might be used by an intermediate level of productive nanosystem stay around longer than the well biodegradable organic molecules. Bio-minerals already are present in enormous masses. These are materials where nature knows how to deal with them.
Possible problems may be:
- High salt concentration (various salts meant here not NaCl) when large amounts are dumpt at one place
- The volumes of bio-mineral production and recycling issues are yet hard to predict.
Technology Level III
In advanced productive nanosystems that is diamondoid nanofactories
the situation worsens again. We are moving from bio-minerals to highly inert gemstones.
Possible problems may be:
- gemstones (e.g. Diamond) e.g. don't really decay. This is good for engineering but bad for nature. Nature is used to deal with large chunks of gemstones. Advanced nanosystems will contain nanoscale gemstones (here called crystolecules) though that may come loose in bad system designs. There might be some exotic microorganisms capable of degrading diamond (TODO: research that) and they might start to evolve with massive availability of diamond but much more likely is that we will much sooner start to attack our own stuff.
Read more on recycling of advanced nanosystems further down.
Biodegradable diamondoid products
For lower performance applications (that is most applications) the probably earlier accessible materials that are significantly weaker than diamond may be sensible to keep even in the more advanced products. Some examples are periclase calcite/aragonite and quartz.
It is not yet clear whether advanced atomically precise products can be made exclusively out of these biodegradable materials while retaining similar density of nanomechanical structures to products out of diamond. The main issues with these materials are:
- sliding surfaces of crystolecules (for bearing applications) made from this materials have not yet been simulated. Ionic bond character is likely to make sliding interfaces harder to design or even impossible. Extremely ionic materials like stone salt could still be used as structural materials.
- If the product continuously decays it needs a sacrificial protection layer facing the environment. It is not yet clear whether a metamaterial hull can be designed that seals the interior is flexible and does not gunk up too badly when some parts are dissolved and recrystallized all over the place. A biodegradable gem-gum-skin e.g. "limestone rubber". If that works one can begin to think about fantastic things like active hull regeneration.
There seem to be no diamondoid carbon based materials (excluding 2D graphite) that decay in reasonable time-spans. (Counterexamples appreciated!) Materials that decay slowly are best. This way non carbon metal cations have time to wash out of organic soil and do not reach problematic concentrations like it is e.g. the case of the use of thawing-salt NaCl for de-icing.
Including non degradable parts in degradable matrix will lead to a situation where remnant "bones" or worse release of persistent nano-particles occur. See: Mobility prevention guideline
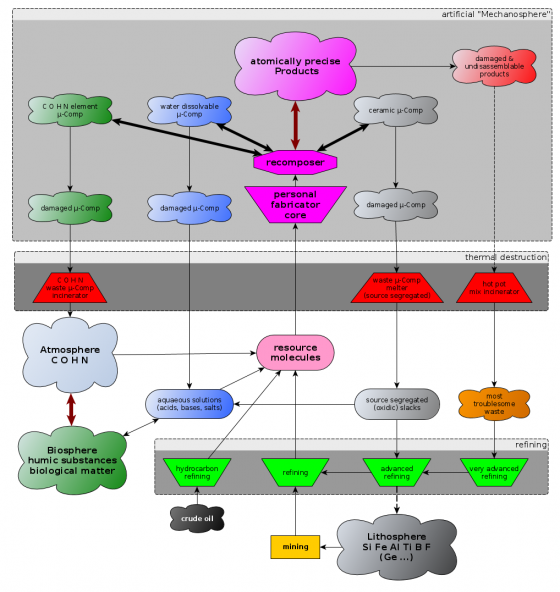
Recycling of diamondoid AP structures
Usage of microcomponents
Diamondoid Mechanosynthesis is an irreversible process. Once a DME is assembled it can not be taken apart again (see atomically precise disassembly). The only way for the bound carbon back to the biosphere is by burning it at sufficiently high temperatures (See the speculative: "hot gas phase recycling cycle"). What will help alleviating this problem is the organisation of APM products into microcomponents (which are quite a bit bigger than DMEs) that can reversibly be joined together and thus can potentially be reused and recomposed. More about those microcomponents can be found on the "assembly levels" page. Microcomponents only need to run through the upper basic assembly levels of a nanofactory (microcomponent recomposer) to get recomposed to a different product.
Tagging microcomponents can help to successfully salvage microcomponents from macroproducts that became singed or broken with random fracture plane.
Inter microcomponent joints that do not destroy themselves (or some of the involved microcomponents) when ruptured are preferable in most applications where maximum strength isn't a necessity (splinter prevention). For this either trivial sticking of coplanar surfaces (Van der Waals force) or specially designed controlled breakage locking mechanisms suffice. If the joints are too weak and do not break in big chunks collectively (through whatever implemented mechanism) rub-off microcomponent dust may be an health issue.
Influence of nanofactory design on recycling
If a nanofactory does vacuum lockout from seperate compartments at the soonest possible moment in the convergent assembly chain - that is as soon as all open radicals are closed - the products are enforced to consist of microcomponents which are potentially recylable by recomposition into other patterns. The downside is that you have to deal with dirt.
If a nanofactory does convergent assembly right up to the full product size it can (it does not necessarly need to but it makes sense) delay the final vacuum lockout right to the very end of the production. If intermediate vacuum lockout layers are omitted the product might be a monolithic diamondoid block that can't be recycled at all and is probably hard to burn too. The reason why this is attractive for developers is that you can simplify design when you don't have to deal with dirt and grit.
One could call a nanofactory that is a failure in this regard an "eternal waste brick nanofactory" if such a design is the first one to spread massively it could mean disaster.
Reversibility of larger scale connection mechanisms ist a necessity for recycling. The original fir tree interlocking expanding ridge joint connection mechanism design by Eric Drexler is irreversible. A reversible version would be highly desirable.
Reuse of microcomponents
Assuming a speculative global microcomponent redistribution system will come into existence then for big immobile objects beyond the weight of an adult person (like buildings) it may be possible to "suck" them away if they are no longer needed. For everyday sized objects running arround having them physically tethered to such a network will often not be possible (e.g. backpacks, drinking cans).
Preference of machine phase
The mobility prevention guideline.
Beside being a necessity for APM in all technology levels but t.level 0 keeping everything in machine phase also prevents spill of AP micro- and nanoparticles (that is microcomponents and DMEs) in the envirounment. The rule to never let go of diamondoid products (never let them escape the machine phase) to keep the biosphere clean obviously has to be dropped at some size level arond the millimeter scale though. In many cases its convenient when makro products come preassembled (laptop) but there are also cases where finding the most pleasing form of assembly is most intuitive and easiest done by hand (art). Such manual assembly of diamondoid AP products will maybe be doable by e.g. (speculative) quasi welding.
- semi-intelligent microcomponent metamaterial designed to allow abrasion only in big chunks ?
- Soft cables and sheets
Bringing carbon back to the biosphere
It's not easy but there is motivation to create specialized equipment for the synthetisation of molecules that are edible by humans. Since many other species will be able to digest them too carbon (e.g. in ethyne form) could be gently spliced back into the biosphere that way.
General
Advanced well designed AP Technology will probably greatly reduce the amount of waste that escapes in the environment. (Speculative) The most critical time is maybe when technology level III arrives but is not yet advanced. Recognizing and cropping out nests of damaged microcomponents will be a rather nontrivial problem. See "self repairing systems".
Production of waste that is irrecoverable at its production time is unavoidable.
Even nature lacking human influence faces this problem. See: great oxygenation event (wikipedia) and the accumulation of lignin in the carboniferous period until fungi figured out how to break it down (wikipedia)
What we can do is to try to limit the rate of irrecoverable waste production to such low levels that technology is likely to catch up to that challenge before the pollution grows beyond all bounds. Something like a dynamic equilibrium. (Is there a general principle waste removal capability always lags behind waste production capability? - Just guessing.)