Intercrystolecular levitation
(wiki-TODO: Add an intro.)
Contents
[hide]Omnidirectionally attractive bearings
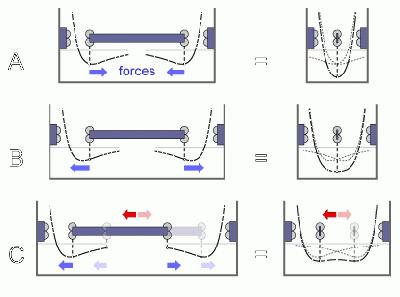
Unlike with macroscopic magnets
When crystolecules (or nanoscale surfaces in general) approach each other
there is a maximal attractive force distance to full surface to surface contact
(that shall be defined as the energy minimum here)
after which the attractive force shrinks again.
This is unlike magnets attracted for ferromagnets or other magnets at the macroscale
where attractive forces only go mootonically up until a very sharp full macroscopic contact.
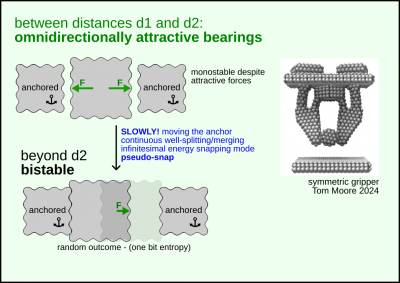
At the nanoscale due to the weakening of the attractive forces on close distances
it is possible to make bearings that levitate a part in such a way
that all forces are are attractive but mutually compensating. Or zero.
Related: Nonmonotonicity of nonbonded forces.
See main page: Negative pressure bearings
Stable electrostatic levitation
(TODO: This needs more analysis and discussion. Especially about in how far Earnshaws theorem applies here.)
(wiki-TODO: this may deserve it's on infographic too eventually.)
This is in analogy to ultra cold atom ion crystals short before Bose Einstein condensation. An ion Coulomb crystal.
Just that charged crystolecules are levitated instead.
Side-note: Laser-cooling of charged crystolecules (just like with atoms) should be possible.
This is more far range levitation though.
A big difference is that unlike free floating single atoms crystolecules have non-degenerate 3D shape
(3 main aces of reference coordinate system specifyable) and thus
their rotational orientation can be fully constrained beside merely their position.
That is: Given the electrodes are close enough to make sufficiently anisotropic local electric fields.
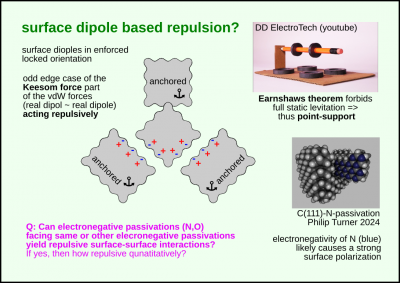
Surface dipole based repulsion
This is an odd edge case of the van der Waals force
and the only one that can act acts repulsively.
Specifically the Keesom force subset of the der Waals forces.
Interacting between two real (non-virtual) dipole pairs.
In biological nanosystems (molecular biology / proteins)
dipoles are rarely forced into a mutually repulsive orientation
and while that may still happen sometimes in enzymes and such
even more dipoles are collectively forced in a repulsive orientation such that forces sum up.
In the case of crystolecules it might be possible though to intentionally force such situations.
Surface passivization on crystolecules with elecronegative elements like nitrogen or oxygen
will create dipoles that are forcibly fixed in their orientation in space.
Repulsive alignment may be enforced by e.g.an piston in cylinder arrangement.
Or any other sort of arrangement really.
One mice example being pencil on magnet flotation toys.
This one also illustrates a fundamental limitation.
There is no static levitation possible.
At least one point needs to be supported.
Reason being Earnswahs theorem.
Net neutral things can't be statically levitated.
(TODO: Some questions deserve more concrete answers.)
❓Q: Can electronegative passivations (N,O) facing same or other elecronegative passivations yield repulsive surface-surface interactions?
If yes, then how repulsive qunatitatively?
Related
- Intercrystolecular interactions
- Intercrystolecular snapping modes
- Intercrystolecular levitation < you are here
External links
Wikipedia: