Difference between revisions of "Surface interface"
m |
(→Related: added link to yet unwritten page Boron nitrogen dative bond interfaces) |
||
| (31 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | {{site specific definition}} | ||
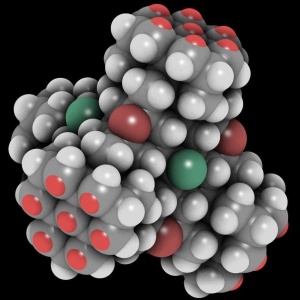
| + | [[File:Tetrapod-openconnects display large square.jpg|300px|thumb|right|The bright red dots in groups of seven are open bonds forming four surface interfaces (some occluded) on this tetrahedric DMSE.]] | ||
| − | + | '''Surface interfaces''' are surfaces of a diamondoid molecular element ([[diamondoid molecular element|DME]]) that have several adjacent (open/unpassivated/unplugged/dangling) covalent bonds (aka radicals) and those bonds are arranged in a way that allows to fuse the DME with a complementary surface interface of an other DME of same or other kind. | |
| − | + | ||
| − | + | ||
| − | + | Surface interfaces are useful in structural DMEs but can also be used fuse fractions of bigger machine DMEs together to a whole DMME. | |
| − | + | This forms a continuum to structural material of a bigger scale machine element. | |
| − | + | ||
| + | The term '''sinterfaces''' could be used as a shorthand for surface interfaces. | ||
| + | it might be misleading though since ''sintering'' is a totally unrelated thermal non atomically precise process. | ||
| + | |||
| + | == matching partners == | ||
| + | |||
| + | The open bonds act (in practical terms) simply as androgynous connection points. | ||
| + | To have anything to assemble | ||
| + | For each surface interface of any [[diamondoid molecular elements|DME]] there must be at least one type of [[diamondoid molecular elements|DME]] that provides a complementary surface interface otherwise there wont be any matching partners which wich the DME can be fused with. | ||
| + | A surface interface and its complement may be identical. Then a DME can fuse with its own type (assuming no geometric obstructions). | ||
Sidenote: If a sinterface lies on a single plane all the bond directions are normal to the plane and the sinterface has at least twofold rotational symmetry then the complementary surface is identical to the original. | Sidenote: If a sinterface lies on a single plane all the bond directions are normal to the plane and the sinterface has at least twofold rotational symmetry then the complementary surface is identical to the original. | ||
| + | |||
| + | == irreversibility / reversibility == | ||
| + | |||
| + | Merging/welding together of two complementary surface intefaces | ||
| + | that have the bonds as tightly packed as the internal structure is an '''irreversible''' process. | ||
| + | This is the case since the formed bonds are indistinguishable from the bonds within the solid | ||
| + | After the connection is formed nothing is left. It's a seamless joint leaving no traces of information how it was connected. | ||
| + | When trying to break the sinterface apart again some random fracture is likely to occur. | ||
| + | |||
| + | Exeptions in which the connection may be '''reversibly''' broken again: | ||
| + | * The bonds formed are more sparse than in the internal structure ['''Todo:''' add infographic] | ||
| + | * The bond is formed at a sharp neck ['''Todo:''' analysis for reliability of this method needed] | ||
| + | |||
| + | Sparse sinterfaces designed to have only few covalent connection points per area and conical widening behind them can be taken apart reversibly. | ||
| + | microcomponents containing such structures need to be moved back into vacuum (possibly down to [[assembly levels|assembly level II]]) if those joints are ought to be disassembled. | ||
| + | |||
| + | == Scissor connection method == | ||
| + | |||
| + | For larger areas correct internal [[positional atomic precision|atomically precise]] bond forming may be an issue [Todo: include reference] | ||
| + | For the best results one can begin to form the bonds from one side then rotating the two parts around the location of the first formed bond(s) closing the remaining wedge of open space in a slowing down scissoring motion while zipping the radicals together at a constant rate. | ||
| + | |||
| + | Note: The zipping speed for radicals is limited by "[[Radical coupling and inter system crossing]]". | ||
| + | |||
| + | == Surface reconstructions == | ||
| + | |||
| + | For some crystallographic surfaces of diamond [https://en.wikipedia.org/wiki/Surface_reconstruction surface reconstruction] is an issue. This has been analyzed ['''Todo:''' add link to nanodiamond study]. The surface reconstructions that are frequently observed today are often caused by heating the sample way above ambient temperature. | ||
| + | When building DMEs [[mechanosynthesis|Mechanosynthesis]] can be done slow enough that such extreme heating does not occur. | ||
| + | This may allow for the creation of more unstable crystallographic surfaces. Whether to use them is another question. | ||
| + | |||
| + | == Size considerations == | ||
| + | |||
| + | When a bunch of DMSEs are merged together enclosing some DMMEs undisassemblable monolithic diamondoid machines are created. | ||
| + | The maximal size in a nanofactory with [[minimal inert vacuum zone]] is a whole [[microcomponents|microcomponent]]. | ||
| + | |||
| + | Note: To merge bigger parts accurately conical alignment pegs can be used ([[Nanosystems]] Figure 14.1.) | ||
| + | |||
| + | == Related == | ||
| + | |||
| + | * '''[[Seamless covalent welding]]''' | ||
| + | * [[technology level III|Advanced atomically precise technology]]. | ||
| + | * [[Boron nitrogen dative bond interfaces]] | ||
| + | |||
| + | == External references == | ||
| + | |||
| + | * [[Nanosystems]] 9.7.3. Covalent interface bonding | ||
| + | |||
| + | [[Category:Technology level III]] | ||
| + | [[Category:Site specific definitions]] | ||
Latest revision as of 11:34, 19 May 2022
Surface interfaces are surfaces of a diamondoid molecular element (DME) that have several adjacent (open/unpassivated/unplugged/dangling) covalent bonds (aka radicals) and those bonds are arranged in a way that allows to fuse the DME with a complementary surface interface of an other DME of same or other kind.
Surface interfaces are useful in structural DMEs but can also be used fuse fractions of bigger machine DMEs together to a whole DMME. This forms a continuum to structural material of a bigger scale machine element.
The term sinterfaces could be used as a shorthand for surface interfaces. it might be misleading though since sintering is a totally unrelated thermal non atomically precise process.
Contents
matching partners
The open bonds act (in practical terms) simply as androgynous connection points. To have anything to assemble For each surface interface of any DME there must be at least one type of DME that provides a complementary surface interface otherwise there wont be any matching partners which wich the DME can be fused with. A surface interface and its complement may be identical. Then a DME can fuse with its own type (assuming no geometric obstructions).
Sidenote: If a sinterface lies on a single plane all the bond directions are normal to the plane and the sinterface has at least twofold rotational symmetry then the complementary surface is identical to the original.
irreversibility / reversibility
Merging/welding together of two complementary surface intefaces that have the bonds as tightly packed as the internal structure is an irreversible process. This is the case since the formed bonds are indistinguishable from the bonds within the solid After the connection is formed nothing is left. It's a seamless joint leaving no traces of information how it was connected. When trying to break the sinterface apart again some random fracture is likely to occur.
Exeptions in which the connection may be reversibly broken again:
- The bonds formed are more sparse than in the internal structure [Todo: add infographic]
- The bond is formed at a sharp neck [Todo: analysis for reliability of this method needed]
Sparse sinterfaces designed to have only few covalent connection points per area and conical widening behind them can be taken apart reversibly. microcomponents containing such structures need to be moved back into vacuum (possibly down to assembly level II) if those joints are ought to be disassembled.
Scissor connection method
For larger areas correct internal atomically precise bond forming may be an issue [Todo: include reference] For the best results one can begin to form the bonds from one side then rotating the two parts around the location of the first formed bond(s) closing the remaining wedge of open space in a slowing down scissoring motion while zipping the radicals together at a constant rate.
Note: The zipping speed for radicals is limited by "Radical coupling and inter system crossing".
Surface reconstructions
For some crystallographic surfaces of diamond surface reconstruction is an issue. This has been analyzed [Todo: add link to nanodiamond study]. The surface reconstructions that are frequently observed today are often caused by heating the sample way above ambient temperature. When building DMEs Mechanosynthesis can be done slow enough that such extreme heating does not occur. This may allow for the creation of more unstable crystallographic surfaces. Whether to use them is another question.
Size considerations
When a bunch of DMSEs are merged together enclosing some DMMEs undisassemblable monolithic diamondoid machines are created. The maximal size in a nanofactory with minimal inert vacuum zone is a whole microcomponent.
Note: To merge bigger parts accurately conical alignment pegs can be used (Nanosystems Figure 14.1.)
Related
- Seamless covalent welding
- Advanced atomically precise technology.
- Boron nitrogen dative bond interfaces
External references
- Nanosystems 9.7.3. Covalent interface bonding