Chemical stability
One often brought up concern about artificial nanomachinery is oxidation. Or more generally corrosion and decay through chemical instability.
This is easily solved by:
- (1) The choice of the right material for the right location.
- (2) Isolation of as much of the inner nanomachinery from the outer environment.
(For systems that allow so. Filters e.g. necessarily have lots of exposed surface.)
Contents
[hide]- 1 Choice of the right material for the right location
- 2 Isolate part of the nano-machinery to allow for chemically more delicate structures
- 3 Highly chemically stable surfaces for housing shells (gem-gum skin)
- 4 Halogen passivated surfaces (Halogenated surfaces)
- 5 Chalkogen and pnictogen passivated surfaces
- 6 Related
Choice of the right material for the right location
(1) Avoid pure metals and metal alloys and use gemstone-like compounds instead. Especially on air exposed surfaces most metals wont work at all. That's well known for iron. But even metals that are air resistant at the macroscale wont work. They form an initially invisibly thin protective oxidation layer (macroscale surface passivation). But on the nanoscale that thin layer can be huge. Much more than a single atomic mono-layer. So small nanoscale components made from metal can when exposed to air oxidize (or otherwise react) through and through. Making them swell up, lose shape, and loose structural integrity. Well there are exceptions. At least gold is immune to attack by air since it's so noble (not willing to give an electron away for a bond). Gold usually does not want any business with oxygen.
Isolate part of the nano-machinery to allow for chemically more delicate structures
It's a similar principle like seen in the case of apples and bananas. But with very different materials. The inside is protected and not exposed to air. Thus it stays non-oxidized. Only in case the inside gets exposed it gets destroyed by oxidation. (Gem-gum systems should usually be built not to break – See: spill prevention guideline, splinter prevention, elasticity emulation, ...)
Delicate tings one would want to isolate inside:
- open covalent bonds / radicals
- surface passivations that could not withstand contact with air
- materials that could not withstand contact with air (most metals and alloys)
Highly chemically stable surfaces for housing shells (gem-gum skin)
Simple hydrocarbons can already be quite stable chemically. E.g. HDPE plastic, which is a very simple hydrocarbon chain can easily cope with concentrated sulfuric acid. Hydrogen passivated diamond surfaces have similar properties. (Moissanite too probably.)
(wiki-TODO: There is a paper on stability of hydrogen passivations on small diamondoid crystals. Find it and reference it here.)
Halogen passivated surfaces (Halogenated surfaces)
Hydrogen is sometimes counted to the halogens since it has with the halogens in common its typical bond order of one. So let's count hydrogen passivated surfaces to the halogenated surfaces here.
The next halogen after hydrogen is fluorine.
- Fluorine F: Fluorated diamond surfaces are known to have extremely high chemical stability.
- Chlorine Cl: Chlorinated diamond surfaces are also still quite stable.
- With heavier halogens (bromine Br, iodine I) surface stability drops.
(Also they are much more rare and thus also much less interesting for structural purposes.)
Forget astatine, it's waay too radioactive. But still, it's half life is likely long enough to permit passivating one single small crystolecule. For research purposes presumably.
The nice thing about halogen passivations is a side effect of their typical bond order being one. Having only one bond to the surface "below" means that a chance of that bond length does (usually) do not lead to a change of stresses and strains in the surface passivation layer. That means different halogens can be swapped for each other without causing part deformations or drops in chemical stability due to too much strained bonds.
Chalkogen and pnictogen passivated surfaces
Swapping atoms out with others that have the same bond order but a different period can chance bond stresses and strains. That may lead to
- chemical destabilization and/or to
- a change of stress-induced part-deformations (= static strains) if the geometry permits that (tubular geometries don't).
E.g. Switching between
- oxygen sulfur selenium (tellurium maybe too metallic) (polonium too radioactive)
- nitrogen phosphorus arsenic (antimony maybe too metallic) (bismuth too metallic)
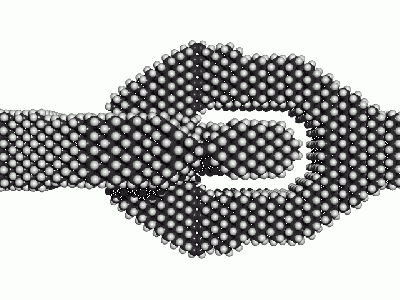
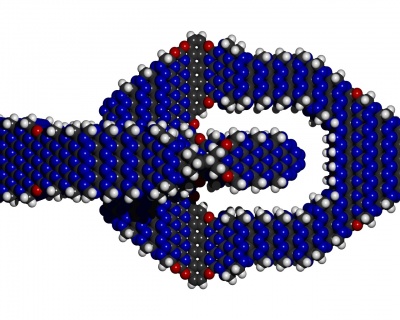
Also hydrogen passivated surfaces can be re-designed for e.g. a nitroxic surface passivation. In fact the software nanoengineer-1 had that implemented in an automated way. See the two similar illustrating images, once hydrogen passivated, once nitroxy passivated. Surface stresses my be significant though. (TODO: Analyze effect of surface stresses in nitroxic passivation on chemical stability). While mechanical stability and thermal stability seem to be well in tact high bond stresses in surface passivations might significantly lower chemical stability against contact with air.
A nitroxy passivation might have the advantage though that the air is also mostly nitrogen and oxygen. Water might act aggressive on these strained bonds though. More analysis needed.
Related
- Chemical stability (this page here)
- Thermal stability
- Mechanical stability
- Pure metals and metal alloys
- Skin – (potentially misleading bio-analogy) – Gem-gum housing shell
- Nanoscale surface passivation
- Macroscale surface passivation