Difference between revisions of "Surface interface"
m |
m |
||
| Line 2: | Line 2: | ||
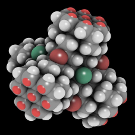
[[File:Wiki-tetrapod-openconnects-black-135.png|frame|The bright red dots in groups of seven are open bonds forming four surface interfaces (some occluded) on this tetrahedric DMSE.]] | [[File:Wiki-tetrapod-openconnects-black-135.png|frame|The bright red dots in groups of seven are open bonds forming four surface interfaces (some occluded) on this tetrahedric DMSE.]] | ||
| − | The term '''surface interfaces''' (or short '''sinterfaces''') may be used for the unpassivated surfaces of [[diamondoid molecular elements|DMSEs]]. | + | The term '''surface interfaces''' (or short '''sinterfaces''') may be used for the unpassivated surfaces of [[diamondoid molecular elements|DMSEs]] (of [[technology level III]]). |
Unpassivated means that open unsaturated bonds (chemical radicals) are present. Those act (in practical terms) simply as androgynous connection poiunts. | Unpassivated means that open unsaturated bonds (chemical radicals) are present. Those act (in practical terms) simply as androgynous connection poiunts. | ||
To have anything to assemble for each surface interface of an [[diamondoid molecular elements|DMSE]] there muts be at least one type of [[diamondoid molecular elements|DMSE]] that provides at least one complementary surface interface. Since the formed bonds are indistinguishable from the bonds within the solid merging/welding together of two complementary sintefaces is an irreversible process. When trying to break the sinterface apart again some random fracture will occur. | To have anything to assemble for each surface interface of an [[diamondoid molecular elements|DMSE]] there muts be at least one type of [[diamondoid molecular elements|DMSE]] that provides at least one complementary surface interface. Since the formed bonds are indistinguishable from the bonds within the solid merging/welding together of two complementary sintefaces is an irreversible process. When trying to break the sinterface apart again some random fracture will occur. | ||
Revision as of 15:19, 29 December 2013
The term surface interfaces (or short sinterfaces) may be used for the unpassivated surfaces of DMSEs (of technology level III). Unpassivated means that open unsaturated bonds (chemical radicals) are present. Those act (in practical terms) simply as androgynous connection poiunts. To have anything to assemble for each surface interface of an DMSE there muts be at least one type of DMSE that provides at least one complementary surface interface. Since the formed bonds are indistinguishable from the bonds within the solid merging/welding together of two complementary sintefaces is an irreversible process. When trying to break the sinterface apart again some random fracture will occur.
For some crystallographic surfaces of diamond surface reconstruction is an issue. This has been analyzed [Todo: add link to nanodiamond study]. The surface reconstuctions that are frequently observed today are often caused by heating the sample way above ambient temperature. When building DMSEs Mechanosynthesis can be done slow enough that such extreme heating does not occur. This may allow for the creation of more unstable crystallographic surfaces. Wether to use them is another question.
Sidenote: If a sinterface lies on a single plane all the bond directions are normal to the plane and the sinterface has at least twofold rotational symmetry then the complementary surface is identical to the original.