Difference between revisions of "Seamless covalent welding"
(→Related: added link to yet unwritten page Surface passivation) |
(→Related: Seamfull covalent welding) |
||
| (19 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
---- | ---- | ||
{{Template:Site specific definition}} | {{Template:Site specific definition}} | ||
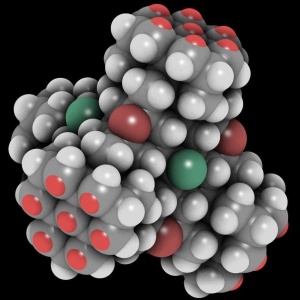
| − | + | [[File:Tetrapod-openconnects display large square.jpg|300px|thumb|right|An example of a structural [[crystolecule]] (or "diamondoid molecular structural element"). The bright red spots in groups of seven are open bonds. These can be seamlessly covalently welded together with a matching pattern.]] | |
| − | + | ||
| − | The | + | |
| − | + | ||
| − | + | This refers to the ideas presented in: [[Nanosystems]] 9.7.3. ''Covalent interfacial bonding'' <br> | |
| + | Not to confuse with: [[Macroscale active align-and-fuse connectors]]. | ||
| + | ---- | ||
| − | + | When [[mechanosynthesis|mechanosynthesizing]] [[crystolecule]]s one can leave open chemical bonds (aka radicals, dangling bonds) on the crystolecules surfaces. <small>(Well, one can leave radicals in the interior too, but that's not of interest in the following discussion).</small> One can include one or more larger patches of such dangling bonds. It is necessary though to take care of possible modes of undesired [[surface reconstruction]]s. | |
| − | * | + | |
| + | If designed well these patches can forms interfaces for "seamless covalent welding". | ||
| + | All other crystolecule types that somewhere on their surface feature a compatible (that is two surfaces with complementary bonds facing each other) welding interface and have a shape that would not overlap with the base crystolecule (the one we analyze) can be permanently and (usually irreversibly) fused together. | ||
| + | |||
| + | * Dangling sigma bonds sticking straight out on a diamond or silicon 111 surface probably work well. | ||
| + | * To prevent several issues (...) when all bonds form simultaneously, welding crystolecules together in a hinge like motion is likely desirable. | ||
| + | * When the weld is located at a sharply necking location it bight be possible to have reversible cleavage exactly where the bond was formed. | ||
| + | |||
| + | == Existence of surface passivations that can preventing welding == | ||
| + | |||
| + | For some if not many or even most [[gemstone like compound]]s (especially strongly ionic ones and rather metallic ones) a highly stable [[surface passivation]] (like present in hydrogen terminated diamond surfaces) may not be possible. | ||
| + | |||
| + | In the worst case all the attempted [[surface passivation]]s would diffuse around at room temperature. | ||
| + | |||
| + | In the better case when diffusion does not happen but the passivation is still weak, then | ||
| + | when the combination of surface to surface compression and temperature gets over some critical level thermo-chemo-mechanically driven rearrangement processes (surface reconstruction of one or both of the each other facing surfaces) may happen. E.g. the surfaces may somehow shove the passivation away to the "side" into more or less defined interstitial positions leading to a probably undesired (weak) weld. | ||
| + | |||
| + | Leaving the surface passivation out to begin with one has fully non-passivated surfaces that will "bond on contact" always forming seamless covalent welds on contact. Right? | ||
| + | |||
| + | {{todo|Investigate whether in ionic compounds motion restraints can be used to force same charges to face each other, such that welding is prevented. Preventing approaching motion seems simple, but what about allowing sliding motion?}} | ||
| + | |||
| + | Difficulties in surface passivation may restrict the usage of many materials to only: | ||
| + | * non-mechanical functions e.g. electronical and optical | ||
| + | * structural functions (no sliding bearing surfaces) | ||
| + | * background filling functions (right behind surface bearing surfaces) - considering a thick shell not a surface passivation | ||
| + | |||
| + | '''Consequences of the "most materials are difficult to prevent from welding together at the nanoscale" issue on recycling:''' | ||
| + | |||
| + | Finding a well passivatable material that does degrade would be highly desirable because:<br> | ||
| + | Diamond (the fist gemstone like material where strong [[surface passivation]] was known to be possible) does not really degrade when left alone as waste in nature (escaping the [[recycling]] process), filling the background behind sliding interfaces with degradable material is a bad idea. If that background is eroded away left over are persistent nanoscale diamond "skin flakes" that are likely very damaging to the environment (food chain). Even a solid non-degradable block of diamond would be better than that. | ||
| + | |||
| + | Related: [[Soil pollutant]] | ||
| + | |||
| + | {{todo|Investigate how well hydrogen passivation on silicon does. On quartz (SiO<sub>2</sub>) what one naturally observes is -OH passivation (at a sparse spacing since quartz with its -o- bridges between Si atoms forms big loops thus has big voids). Probably not very suitable for sliding interfaces.}} | ||
| + | |||
| + | == Notes == | ||
| + | |||
| + | * {{wikitodo|add existing illustrative graphic of opposing surfaces with open bonds matching up}} | ||
== Related == | == Related == | ||
| − | * [[Connection method]] | + | * '''[[Surface interface]]''' |
| + | * '''[[Seamfull covalent welding]]''' | ||
| + | * Not to confuse with: [[Macroscale active align-and-fuse connectors]] | ||
| + | * Up: [[Connection method]] | ||
* [[Surface passivation]] | * [[Surface passivation]] | ||
| + | * [[Adhesive interfaces]] | ||
| + | * "welding" different gemstones seamlessly covalent together: [[Organic anorganic gemstone interface]] | ||
| + | ---- | ||
| + | * [[Boron nitrogen dative bond interfaces]] | ||
| + | ---- | ||
| + | * Not to confuse with: [[Macroscale active align-and-fuse connectors]] | ||
| + | |||
[[Category:Technology level III]] | [[Category:Technology level III]] | ||
[[Category:Site specific definitions]] | [[Category:Site specific definitions]] | ||
Latest revision as of 13:44, 11 October 2023
This refers to the ideas presented in: Nanosystems 9.7.3. Covalent interfacial bonding
Not to confuse with: Macroscale active align-and-fuse connectors.
When mechanosynthesizing crystolecules one can leave open chemical bonds (aka radicals, dangling bonds) on the crystolecules surfaces. (Well, one can leave radicals in the interior too, but that's not of interest in the following discussion). One can include one or more larger patches of such dangling bonds. It is necessary though to take care of possible modes of undesired surface reconstructions.
If designed well these patches can forms interfaces for "seamless covalent welding". All other crystolecule types that somewhere on their surface feature a compatible (that is two surfaces with complementary bonds facing each other) welding interface and have a shape that would not overlap with the base crystolecule (the one we analyze) can be permanently and (usually irreversibly) fused together.
- Dangling sigma bonds sticking straight out on a diamond or silicon 111 surface probably work well.
- To prevent several issues (...) when all bonds form simultaneously, welding crystolecules together in a hinge like motion is likely desirable.
- When the weld is located at a sharply necking location it bight be possible to have reversible cleavage exactly where the bond was formed.
Existence of surface passivations that can preventing welding
For some if not many or even most gemstone like compounds (especially strongly ionic ones and rather metallic ones) a highly stable surface passivation (like present in hydrogen terminated diamond surfaces) may not be possible.
In the worst case all the attempted surface passivations would diffuse around at room temperature.
In the better case when diffusion does not happen but the passivation is still weak, then when the combination of surface to surface compression and temperature gets over some critical level thermo-chemo-mechanically driven rearrangement processes (surface reconstruction of one or both of the each other facing surfaces) may happen. E.g. the surfaces may somehow shove the passivation away to the "side" into more or less defined interstitial positions leading to a probably undesired (weak) weld.
Leaving the surface passivation out to begin with one has fully non-passivated surfaces that will "bond on contact" always forming seamless covalent welds on contact. Right?
(TODO: Investigate whether in ionic compounds motion restraints can be used to force same charges to face each other, such that welding is prevented. Preventing approaching motion seems simple, but what about allowing sliding motion?)
Difficulties in surface passivation may restrict the usage of many materials to only:
- non-mechanical functions e.g. electronical and optical
- structural functions (no sliding bearing surfaces)
- background filling functions (right behind surface bearing surfaces) - considering a thick shell not a surface passivation
Consequences of the "most materials are difficult to prevent from welding together at the nanoscale" issue on recycling:
Finding a well passivatable material that does degrade would be highly desirable because:
Diamond (the fist gemstone like material where strong surface passivation was known to be possible) does not really degrade when left alone as waste in nature (escaping the recycling process), filling the background behind sliding interfaces with degradable material is a bad idea. If that background is eroded away left over are persistent nanoscale diamond "skin flakes" that are likely very damaging to the environment (food chain). Even a solid non-degradable block of diamond would be better than that.
Related: Soil pollutant
(TODO: Investigate how well hydrogen passivation on silicon does. On quartz (SiO2) what one naturally observes is -OH passivation (at a sparse spacing since quartz with its -o- bridges between Si atoms forms big loops thus has big voids). Probably not very suitable for sliding interfaces.)
Notes
- (wiki-TODO: add existing illustrative graphic of opposing surfaces with open bonds matching up)
Related
- Surface interface
- Seamfull covalent welding
- Not to confuse with: Macroscale active align-and-fuse connectors
- Up: Connection method
- Surface passivation
- Adhesive interfaces
- "welding" different gemstones seamlessly covalent together: Organic anorganic gemstone interface
- Not to confuse with: Macroscale active align-and-fuse connectors