Nanoscale surface passivation
Old page: Surface passivation
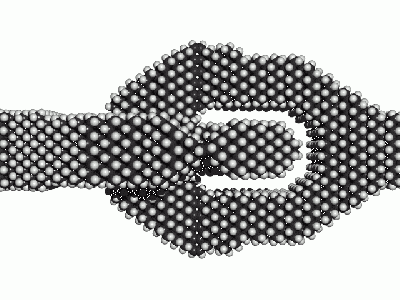
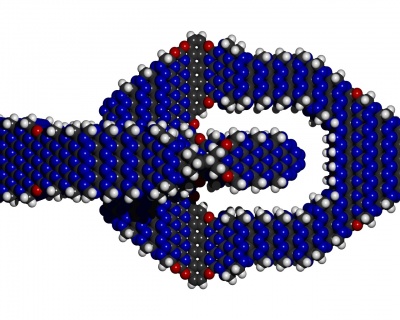
Intuitively passivation is like the flour on the bread-dough that one adds such
that it does not stick together with itself and just about anything.
Also it prevents material surfaces from oxidizing. More on that later.
- Surfaces of crystolecules that are supposed to come in contact with other surfaces need to be passivated in order to not fuse together irreversiby .
- Surfaces of crystolecules that are supposed to come in contact with air need passivation.
(well, first air contact may be used for passivation of non-sliding surfaces but the result may be somewhat random)
Up: Passivation (disambiguation)
(wiki-TODO: eventually merge in the old duplicate page Surface passivation)
Contents
[hide]Protection against oxidation
This is not as important as it sounds.
Most of the materials that are especially suitable for gemstone metamaterial technology (gemstone like compounds)
are already oxidic in nature, fully odidized, or highly inert towards air.
The whole nanoscale parts (crystolecules) are often made from the same material as a macroscopic (but thin) passivation layer so to say.
Nanoscale passivation is mostly not meant to prevent oxidation but rather meant to:
- prevent irreversible stick together and
- provide smooth sliding surfaces for superlubricity.
Also only a very very small fraction of nano structures in gemstome metamaterial products are exposed to air.
Even less so for moving active nanomachinery.
Most nanostructures are well sealed inside the products.
So the vast majority of nano structures that resides inside the products and nerver comes into contact with air
allows for a much bigger design space in choice of base material and eventual passivation.
Itnuiton: An apple or a babana does not get brown unless you cut them open.
passivation of diamond, lonsdaleite and other allotropes of carbon
Diamond and it's allotropes are best passivated with hydrogen.
This works very well and makes vers stable surfaces. We know that from hydrogarbon chains that make up
gasoline and all of our current day plastics.
Passivation of silicon containing gemstone like compounds
In the case of pure silicon the bigger size of silicon atoms already makes for more pronounced surface corrugations in case of a hydrogen passivation.
This makes designs for superlubricating surface interaction harder.
Increased corrugation already starts with moissanite (SiC) where only every second atom is a silicon.
A simple hydrogen passivation is less stable than in the case of carbon.
For silicon OH groups seem to be still quite stable.
As one can see from the highly stable Si-O bonds in polymer chains of current day available silicones.
The perhaps more stable hydroxy passivations are probably pretty bad for sliding interfaces because:
- Hydroxy passivations (·OH) are big singly bonded protrusions prone to snapback.
- With hydroxy passivations (·OH) being angled there is a unconstrained degree of freedom.
This may serve as an additional pathway for energy dissipation.
Surface corrugation really becomes terrible when going to sparsely filled oxide minerals like quartz (SiO2).
These have much bigger voids inside than more compact gemstone like compounds.
Plus they especially like hydroxy passivations.
Options for these more sparse materials:
- use them just for structural purposes
- give them some sort of much smoother surface cover like graphene maybe. Question is: How to "tack" it on neatly and tightly?
- look for similar less sparse materials stishovite instead of quartz (both polymorphs of Silicon dioxide SiO2
Passivations for transition metal monoxides
Maybe these could be covered with graphene?
See: Sandwich compound
Passivation by "graphene sheet lining"
It may be possible to passivate some base materials by tacking on graphene sheets onto the surface.
The bonds found in sandwich compound may be usable here.
Especially for materials that are otherwise hard to passivate this may be a possible option.
Graphene sheet lining may be an option for bigger sized gears
where teet are no longer single atoms but teeth instead already approximate evolvent or cycloid profiles.
Concerns:
- Can the tack-on density by high enough such that between the tack-ons there is not too low of a stiffness?
- Will a too dense tack-on pattern distort the graphenes electronic structure so much that it will become too reactive or even fully unstable?
- How well does the graphene conform to the underlying material?
- How much curvature is ok before localized kinks or too much change in electronic structure?
- How well can the graphene smooth out steps below in the underlying material?
- ... and so on and so forth ...
Related
- Chemical stability
- Macroscale surface passivation – Passivation layer mineral
- Passivation (disambiguation)
- Passivation bending issue – crystolecule deformations (strains) induced by undesired stresses caused by passivations
- Passivation layer mineral – This is about macroscale passivation. At the nanoscale passivation layer minerals still need passivation to not fuse together.
- Seamless covalent welding
