Difference between revisions of "Snapback"
(→External links: added complementary link) |
m (→Related: added * Stiff cantilever AFM) |
||
| (5 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Stub}} | {{Stub}} | ||
| + | [[File:0322pairSnap.gif|thumb|400px|Citation:"Softly supported sliding atoms can undergo abrupt transitions in energy" <ref name="unsmooth">'''Eric Drexlers former homepage (webarchive):''' [https://web.archive.org/web/20160314100841/http://e-drexler.com/p/04/02/0315pairSnap.html Softly supported sliding atoms can undergo abrupt transitions in energy]</ref>]] | ||
| + | |||
An energy dissipation mechanism. | An energy dissipation mechanism. | ||
| Line 31: | Line 33: | ||
Just that here energy mostly initially get dissipated into sound energy. | Just that here energy mostly initially get dissipated into sound energy. | ||
| + | == Experimentally == | ||
| + | |||
| + | As of 2024 in [[stiff cantilever AFM]] CO molecules have been picked up and <br> | ||
| + | used for imaging graphic molecules to subatomic resolution. <br> | ||
| + | The linear CO molecules are weak against sideways bending though. <br> | ||
| + | This leads to image artifacts making bonds look sharper than they really are (in terms of pauli-repulsion-shape). <br> | ||
| + | Crossing a bond between graphic rings too closely there might be some snap back going on. <br> | ||
| + | Albeit no artifacts like sudden steps are visible in the published images. <br> | ||
| + | Snap-backs would be many many orders of magnitude faster than the scanning motion of the tip. <br> | ||
| + | Bending distortions can also be attractive not only repulsive. <br> | ||
| + | On the outer border the bending of the CO molecule might rather gradually circle around rather than snap. <br> | ||
| + | |||
== Related == | == Related == | ||
| Line 36: | Line 50: | ||
* Snapback exactly what one does not want when designing for [[superlubricity]] | * Snapback exactly what one does not want when designing for [[superlubricity]] | ||
* [[Snap connectors]] | * [[Snap connectors]] | ||
| + | * [[Molecular dynamics implementation cheat sheet]] – sideward bond bending stiffness is only 1/20th of radial bond stretching/compressing stiffness | ||
| + | * [[Stiff cantilever AFM]] | ||
== External links == | == External links == | ||
| − | * Archived page from Eric Drexlers fomer website: <br>[https://web.archive.org/web/20160314100841/http://e-drexler.com/p/04/02/0315pairSnap.html Softly supported sliding atoms can undergo abrupt transitions in energy] | + | * Archived page from Eric Drexlers fomer website: <br>'''[https://web.archive.org/web/20160314100841/http://e-drexler.com/p/04/02/0315pairSnap.html Softly supported sliding atoms can undergo abrupt transitions in energy]''' |
* Complementary to that: <br>[https://web.archive.org/web/20160314060004/http://e-drexler.com/p/04/02/0315pairPot.html Stiffly supported sliding atoms have a smooth interaction potential] | * Complementary to that: <br>[https://web.archive.org/web/20160314060004/http://e-drexler.com/p/04/02/0315pairPot.html Stiffly supported sliding atoms have a smooth interaction potential] | ||
| + | |||
| + | == References == | ||
| + | |||
| + | <references/> | ||
Latest revision as of 21:52, 25 February 2024
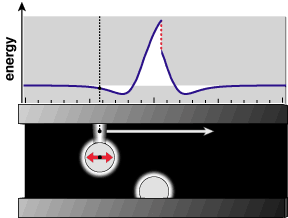
An energy dissipation mechanism.
When a part of nanoscale surface passivation A that sticks out
- gets pulled to the side and tensioned like a spring by an opposing part B,
- then slides past this opposing part B
- then swings back and forth unconstrainedly
- then looses its energy to vibrations of atoms that it is bonded to below.
Contents
Snapback either designed for or designed against
One will usually want to design reusable molecular machine elements such that they
either maximize snapback or avoid snapback all together by large margin.
Orthogonal set of mechanical components.
When designing for superlubrication snapback is highly undesired.
Energetically
This can be seen as a fusion of potential energy well
where the bottoms are not brought on the same height first.
The following dissipation is not visible in this model.
Bigger nanoscale snapback
Crystolecule clips that do not provide means for energy recuperation snap back. Larger size scales gives less energy loss from snapbacks.
Macroscopic analogy
Making a ruler vibrate on the corner of a desk.
Just that here energy mostly initially get dissipated into sound energy.
Experimentally
As of 2024 in stiff cantilever AFM CO molecules have been picked up and
used for imaging graphic molecules to subatomic resolution.
The linear CO molecules are weak against sideways bending though.
This leads to image artifacts making bonds look sharper than they really are (in terms of pauli-repulsion-shape).
Crossing a bond between graphic rings too closely there might be some snap back going on.
Albeit no artifacts like sudden steps are visible in the published images.
Snap-backs would be many many orders of magnitude faster than the scanning motion of the tip.
Bending distortions can also be attractive not only repulsive.
On the outer border the bending of the CO molecule might rather gradually circle around rather than snap.
Related
- Nanoscale surface passivation
- Snapback exactly what one does not want when designing for superlubricity
- Snap connectors
- Molecular dynamics implementation cheat sheet – sideward bond bending stiffness is only 1/20th of radial bond stretching/compressing stiffness
- Stiff cantilever AFM
External links
- Archived page from Eric Drexlers fomer website:
Softly supported sliding atoms can undergo abrupt transitions in energy - Complementary to that:
Stiffly supported sliding atoms have a smooth interaction potential
References
- ↑ Eric Drexlers former homepage (webarchive): Softly supported sliding atoms can undergo abrupt transitions in energy