Difference between revisions of "Air as a resource"
(→Main compounds: some improvements -- added subchapters) |
m (/* Traces */ ==> →Trace gasses) |
||
| Line 36: | Line 36: | ||
Structures of pure solid (sp<sup>3</sup>) nitrogen can be produced but they are highly explosive. (Easy production of explosives is one of APM's [[dangers]].) | Structures of pure solid (sp<sup>3</sup>) nitrogen can be produced but they are highly explosive. (Easy production of explosives is one of APM's [[dangers]].) | ||
| − | == | + | == Trace gasses == |
=== Sulfur === | === Sulfur === | ||
Revision as of 10:22, 20 August 2020
Air can directly be used as building material since with its constituents carbon dioxide (CO2) water (H2O) and nitrogen gas (N2) one has the elements carbon oxygen hydrogen and nitrogen (C, O, H, N) available. A set that allows the production of solid high performance products.
Air can be used by:
- any AP small scale factory equipped with means of filtereing
- Mobile carbon dioxide collector balloons
Contents
Main compounds
Only carbon
(On earth) the gas that defines the fraction of atmosphere that can be used to produce solid materials (that are not explosive) is carbon dioxide. It is by far the most abundant one of the present gasses that can be used this way. With carbons four bonds it can form highly stable three dimensional networked diamondoid materials.
Other present elements not useful on their own
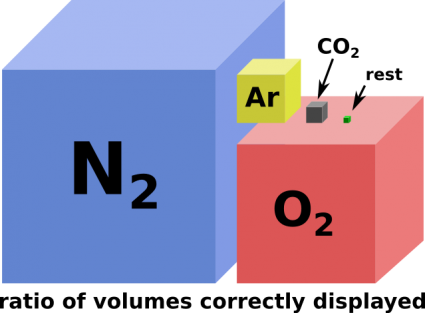
All the types of molecules that are more abundant in the Atmosphere contain only atoms with less bonds. Making solid objects with only these atoms with three or less bonds is either very dangerous or impossible. Pure nitrogen with it's three bonds can be made to form solids at ambient conditions but it is an extremely potent explosive. Pure oxygen with its two bonds at best can be made to form polymeric chains which are even less stable than solid nitrogen. Pure hydrogen with one bond per atom (taken from water vapour) cannot form solids at ambient conditions at all. Argon as a noble gas (also a main component of the atmosphere) does not form bonds at all. (As a side note: Argon may be used to fill Nanofactories at the lowest levels that need a vacuum equivalent environment.) An exception might be water ice which could be considered a building material in some cases.
When one assumes a filtering efficiency near 100% (with advanced AP technology) one can extract around 1kg of carbon per hour with a middle sized blowing machine (of todays technology).
Can we cheating with nitrogen?
When beta carbon nitride (Wikipedia) (which's properties are not well known yet) is used as structural material this mass roughly doubles since four thirds of the used atoms are nitrogen that on its own would not be usable. While probably not explosive this material might still pose a higher fire hazard than pure carbon due to its higher energy content.
Structures of pure solid (sp3) nitrogen can be produced but they are highly explosive. (Easy production of explosives is one of APM's dangers.)
Trace gasses
Sulfur
Whats lacking most are heavier elements. Sulfur for example turned out to be useful for DMME bearings since it has larger diameter than it's cousin oxygen and can thus form deeper grooves. It shouldn't be hard to get along without it though.
Traces of sulfur are present in the form of sulfur hexafluoride (Wikipedia). Industrial activity has elevated the leves from below 4 ppt to above 7 ppt in the last sixteen years. Sulfur dioxide levels in earths atmosphere are around 1ppb (wikipedia)
Other trace gasses
Fluorine: Another trace gas that is encountered in the linde cycle (Wikipedia) is tetrafluoromethane (Wikipedia).
Noble gasses: Further noble trace gasses: Neon Helium Kryptom und Xenon
Dust
There is also dust which beside carbon may bring some traces of sulfur, phosporus, silicon, aluminum, iron and common alkali metals. But dust will need some more advanced pre-possessing (e.g. possibly acidic dissolution for organics).
Notes
The versatility of diamondoid materials and metamaterials are the reason why AP Technology can replace scarce elements with just a few abundant ones.
Related
- Soil as a resource
- Carbon dioxide collector
- Drawing elements usable for the structural core of DMEs like aluminum silicon titanium and more form trace amounts of aerosol dust.
- Living from air and sun
- Abundant elements
Extraterrestrial "airs"
See: Gas giant atmospheres, Venus