Difference between revisions of "Air as a resource"
(→Main compounds: improved) |
m (→Traces) |
||
| Line 36: | Line 36: | ||
Traces of sulfur are present in the form of sulfur hexafluoride ([http://en.wikipedia.org/wiki/Sulfur_hexafluoride Wikipedia]). Industrial activity has elevated the leves from below 4 ppt to above 7 ppt in the last sixteen years. | Traces of sulfur are present in the form of sulfur hexafluoride ([http://en.wikipedia.org/wiki/Sulfur_hexafluoride Wikipedia]). Industrial activity has elevated the leves from below 4 ppt to above 7 ppt in the last sixteen years. | ||
| + | ['''todo:''' check how much trace sulfur dioxide is present] | ||
| + | |||
Another trace gas that is encountered in the linde cycle ([http://en.wikipedia.org/wiki/Hampson%E2%80%93Linde_cycle Wikipedia]) is tetrafluoromethane ([http://en.wikipedia.org/wiki/Tetrafluoromethane Wikipedia]). | Another trace gas that is encountered in the linde cycle ([http://en.wikipedia.org/wiki/Hampson%E2%80%93Linde_cycle Wikipedia]) is tetrafluoromethane ([http://en.wikipedia.org/wiki/Tetrafluoromethane Wikipedia]). | ||
Revision as of 09:03, 24 April 2015
With carbon dioxide CO2 water H2O and nitrogen gas N2 one has hydrogen carbon oxygen and nitrogen (H C O N) available. Air can thus directly be used as building material.
Air can be used by:
- any AP small scale factory equipped with means of filtereing
- air using micro ships
Main compounds
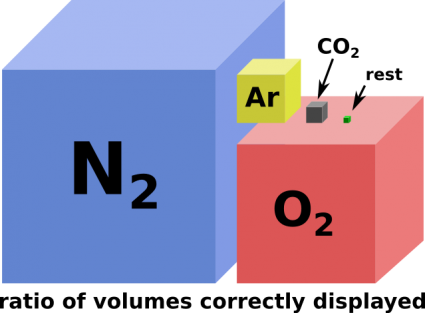
The gas that defines the fraction of atmosphere that can be used to produce solid materials (that are not explosive) is carbon dioxide. It is by far the most abundant one of the present gasses that can be used this way. With carbons four bonds it can form highly stable three dimensional networked diamondoid materials.
All more abundant molecules in the Atmosphere contain only atoms with less bonds. Making solid objects with only them is either very dangerous or impossible. Pure nitrogen with it's three bonds can be made to form solids at ambient conditions but it is an extremely potent explosive. Pure oxygen with its two bonds at best can be made to form polymeric chains which are even less stable than solid nitrogen. Pure hydrogen with one bond per atom taken from water vapor cannot form solids ambient conditions at all. Argon as a noble gas (also a main component of the atmosphere) does not form bonds at all. It may be used to fill Nanofactories at the lowest levels that need a vacuum equivalent environment. An exception might be ice which could be considered a building material in some cases.
When one assumes a filtering efficiency near 100% (with advanced AP technology) one can extract around 1kg of carbon per hour with a middle sized blowing machine (of todays technology).
When beta carbon nitride (Wikipedia) (which's properties are not well known yet) is used as structural material this mass roughly doubles. Structures of pure solid (sp3) nitrogen can be produced but they are higly explosive. (Easy production of explosives is one of APM's dangers.)
Traces
Whats lacking most are heavier elements. Sulfur for example turned out to be useful for DMME bearings since it has larger diameter than it's cousin oxygen and can thus form deeper grooves. It shouldn't be hard to get along without it though.
Traces of sulfur are present in the form of sulfur hexafluoride (Wikipedia). Industrial activity has elevated the leves from below 4 ppt to above 7 ppt in the last sixteen years. [todo: check how much trace sulfur dioxide is present]
Another trace gas that is encountered in the linde cycle (Wikipedia) is tetrafluoromethane (Wikipedia).
Further noble trace gasses: Neon Helium Kryptom und Xenon
Notes
The versatility of diamondoid materials and metamaterials are the reason why AP Technology can replace scarce elements with just a few abundant ones.
Related
- soil as a resource
- Drawing elements usable for the structural core of DMEs like aluminum silicon titanium and more form trace amounts of aerosol dust.
- living from air and sun
Extraterrestrial "airs"
See: Gas giant atmospheres, Venus