Difference between revisions of "Carbon dioxide"
(added just a related section for now) |
(added a bit - work in progress) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{stub}} | {{stub}} | ||
| + | [[file:Atmosphere-composition-639x470.png|thumb|425px|Gasses in Earths atmosphere. Half of that cube representing CO<sub>2</sub> is man made. (~200ppm pre-industrial, ~400ppm and rising as of 2021) [http://apm.bplaced.net/w/images/9/93/Atmosphere-composition.svg SVG] <br> (variable atmospheric humidity omitted) ]] | ||
Carbon dioxide. Our global crisis number one today (2021). <br> | Carbon dioxide. Our global crisis number one today (2021). <br> | ||
It seems that with [[gemstone metamaterial technology]] the problem would be quite easy to fix via various options. <br> | It seems that with [[gemstone metamaterial technology]] the problem would be quite easy to fix via various options. <br> | ||
The big issue though is that it is quite unclear how long it will still take for the technology to emerge and mature. <br> | The big issue though is that it is quite unclear how long it will still take for the technology to emerge and mature. <br> | ||
| + | |||
| + | == Direct air capture == | ||
| + | |||
| + | Due to mixing entropy there is a fundamental energy cost to pay to filter CO2 back out of the atmosphere. <br> | ||
| + | Luckily despite CO<sub>2<sub> being quite dilutely present withing air this energetic cost is small <br> | ||
| + | compared to the energy needed for reducing the carbon (i.e. splitting it off from the oxygen). <br> | ||
| + | Actuall diluteness: 278ppm (~0.000278 to 1) pre-industrially and 417ppm (and rising) today. <br> | ||
| + | |||
| + | {{wikitodo|Find that one document that makes an excellent calculation on that again and link it here.}} | ||
| + | |||
| + | == Carbon sequestration == | ||
| + | |||
| + | === As carbonates - carbonic acid salts === | ||
| + | |||
| + | Most calcium in Earths crust is actually bound in silicates (due to their abundance). <br> | ||
| + | Reacting CO<sub>2<sub> with calcium silicate minearals like wollastonite is actually exergonic. <br> | ||
| + | Problem is getting it up to the ground and crushed or dissolved so the CO<sub>2<sub> can react. <br> | ||
| + | |||
| + | Related: Mineral weathering. | ||
| + | |||
| + | === As oxygen free carbides === | ||
| + | |||
| + | Gem grade silicon carbide [[Moisssantite]] seems a very good candidate as silicon is maximally abundant and <br> | ||
| + | the compound is heavily self limiting combustion by slack formation. Plus it doubles as excellent building material.<br> | ||
| + | |||
| + | Titanium carbide | ||
| + | |||
| + | === Elemental === | ||
| + | |||
| + | Diamond / Graphite / Nanotubes / various allotropes. <br> | ||
| + | The problem here is flammability combined with the gigantic quantities. <br> | ||
| + | Fully oxidized carbonates or heavily compounds that heavily self limit their combustion (e.g. by slack formation) <br> | ||
| + | seem a lot safer for carbon sequestration. | ||
== Related == | == Related == | ||
Latest revision as of 15:37, 9 July 2024
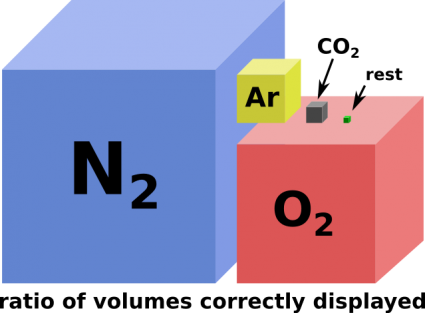
(variable atmospheric humidity omitted)
Carbon dioxide. Our global crisis number one today (2021).
It seems that with gemstone metamaterial technology the problem would be quite easy to fix via various options.
The big issue though is that it is quite unclear how long it will still take for the technology to emerge and mature.
Contents
Direct air capture
Due to mixing entropy there is a fundamental energy cost to pay to filter CO2 back out of the atmosphere.
Luckily despite CO2 being quite dilutely present withing air this energetic cost is small
compared to the energy needed for reducing the carbon (i.e. splitting it off from the oxygen).
Actuall diluteness: 278ppm (~0.000278 to 1) pre-industrially and 417ppm (and rising) today.
(wiki-TODO: Find that one document that makes an excellent calculation on that again and link it here.)
Carbon sequestration
As carbonates - carbonic acid salts
Most calcium in Earths crust is actually bound in silicates (due to their abundance).
Reacting CO2 with calcium silicate minearals like wollastonite is actually exergonic.
Problem is getting it up to the ground and crushed or dissolved so the CO2 can react.
Related: Mineral weathering.
As oxygen free carbides
Gem grade silicon carbide Moisssantite seems a very good candidate as silicon is maximally abundant and
the compound is heavily self limiting combustion by slack formation. Plus it doubles as excellent building material.
Titanium carbide
Elemental
Diamond / Graphite / Nanotubes / various allotropes.
The problem here is flammability combined with the gigantic quantities.
Fully oxidized carbonates or heavily compounds that heavily self limit their combustion (e.g. by slack formation)
seem a lot safer for carbon sequestration.
Related
- Carbon dioxide collector
- Mechanosynthetic carbon dioxide splitting – (Mechanosynthetic water splitting)
- Air as a resource